|
Medicine RSS-Feeds by Alexandros G. Sfakianakis,Anapafseos 5 Agios Nikolaos 72100 Crete Greece,00302841026182,00306932607174,alsfakia@gmail.com
Πληροφορίες
Τρίτη 19 Ιανουαρίου 2021
The role of the glutamine transporter ASCT2 in antineoplastic therapy
Glutaminolysis is a metabolic route essential for survival and growth of prostate cancer cells and a target of 5α-dihydrotestosterone regulation
|
Efficacy of salvage stereotactic radiotherapy (SRT) for locally recurrent brain metastases after initial SRT and characteristics of target population
|
Variant of SNPs at lncRNA NEAT1 contributes to gastric cancer susceptibility in Chinese Han population
|
Brachytherapy boost (BT-boost) or stereotactic body radiation therapy boost (SBRT-boost) for high-risk prostate cancer (HR-PCa)
|
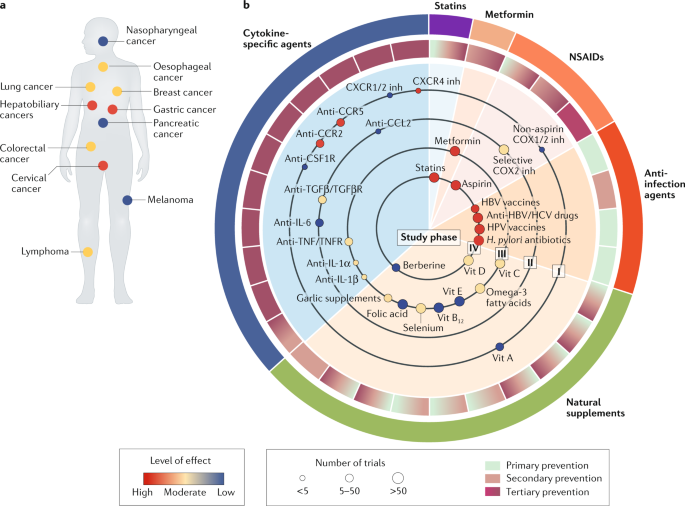
Targeting cancer-promoting inflammation — have anti-inflammatory therapies come of age?
|
Hypoxia-inducible miR-196a modulates glioblastoma cell proliferation and migration through complex regulation of NRAS
|
PD-1 and PD-L2 expression predict relapse risk and poor survival in patients with stage III colorectal cancer
|
Clinical perspectives of BET inhibition in ovarian cancer
|
Protein arginine methyltransferase 5: a potential cancer therapeutic target
|
In silico transcriptomic mapping of integrins and immune activation in Basal-like and HER2+ breast cancer
|
Αρχειοθήκη ιστολογίου
-
►
2023
(366)
- ► Φεβρουαρίου (184)
- ► Ιανουαρίου (182)
-
►
2022
(2814)
- ► Δεκεμβρίου (182)
- ► Σεπτεμβρίου (213)
- ► Φεβρουαρίου (264)
- ► Ιανουαρίου (262)
-
▼
2021
(3815)
- ► Δεκεμβρίου (229)
- ► Σεπτεμβρίου (276)
- ► Φεβρουαρίου (64)
-
▼
Ιανουαρίου
(344)
-
▼
Ιαν 19
(25)
- The role of the glutamine transporter ASCT2 in ant...
- Glutaminolysis is a metabolic route essential for ...
- Efficacy of salvage stereotactic radiotherapy (SRT...
- Variant of SNPs at lncRNA NEAT1 contributes to gas...
- Brachytherapy boost (BT-boost) or stereotactic bod...
- Targeting cancer-promoting inflammation — have ant...
- Hypoxia-inducible miR-196a modulates glioblastoma ...
- PD-1 and PD-L2 expression predict relapse risk and...
- Clinical perspectives of BET inhibition in ovarian...
- Protein arginine methyltransferase 5: a potential ...
- In silico transcriptomic mapping of integrins and ...
- Acetylation-stabilized chloride intracellular chan...
- CHRNA5 belongs to the secondary estrogen signaling...
- The role of capecitabine-based neoadjuvant and adj...
- Tumor volume: a new prognostic factor of oncologic...
- Aglycemic growth enhances carbohydrate metabolism ...
- Comparative analysis of patients with upper urinar...
- Clinical features associated with the efficacy of ...
- Targeting cancer-promoting inflammation — have ant...
- The watch-and-wait strategy versus surgical resect...
- The mutation of BCOR is highly recurrent and oncog...
- Impact of biomarkers and primary tumor location on...
- Biomarker testing and mutation prevalence in metas...
- Appropriateness of trifluridine/tipiracil in the c...
- Nomograms to predict lung metastasis probability a...
-
▼
Ιαν 19
(25)
-
►
2020
(5754)
- ► Δεκεμβρίου (401)
- ► Σεπτεμβρίου (365)
- ► Φεβρουαρίου (754)
- ► Ιανουαρίου (894)
-
►
2019
(146)
- ► Δεκεμβρίου (19)
- ► Σεπτεμβρίου (54)