High-Dose Ipilimumab and High-Dose Interleukin-2 for Patients With Advanced Melanoma:
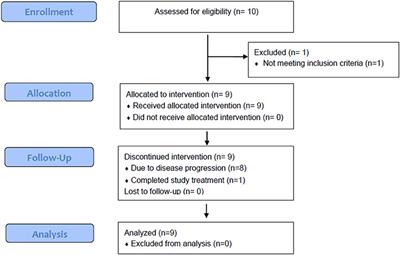
High-dose ipilimumab (IPI) and high-dose interleukin-2 (IL-2) are approved agents for metastatic melanoma, but the efficacy and safety of the combination are unknown. The objective of this study was to evaluate the feasibility, safety, and efficacy of combination high-dose IPI and high-dose IL-2 in patients with histologically confirmed advanced unresectable stage III and IV melanoma. This Phase II, multicenter, open-label, single-arm trial was conducted in nine patients enrolled between 12/2014 and 12/2015. Subjects were treated with high-dose IPI 10 mg/kg intravenous (IV) every 3 weeks for four doses starting at week 1 and high-dose IL-2 (600,000 IU/kg IV bolus every 8 h for up to 14 doses) concurrently with IPI at weeks 4 and 7. After the first 12 weeks of combination therapy, maintenance IPI (10 mg/kg IV) monotherapy was administered every 12 weeks for up to 1 year. No patient had received prior PD-1 blockade, and only one received prior vemurafenib. Confirmed partial response was achieved in one (11%), stable disease in four (44%), and progressive disease in four (44%) of nine patients. Two patients achieved durable disease control of 44+ and 50+ months at the most recent follow-up without subsequent therapy. The median overall survival was not reached after a minimum 24 months of follow-up time. One-year and 2-year survival rates were 89 and 67%, respectively. Seven patients (78%) experienced grade 3 or 4 adverse events related to the study therapy, three of which were attributed to both agents. One patient discontinued the treatment due to liver and kidney toxicity. While toxicity was significant, all events were reversible, and there was no treatment-related mortality. In peripheral blood of patients with decreasing tumor burden, the ratio of the non-classical MHC-II proteins HLA-DM to HLA-DO increased 2-fold, raising the possibility of the ratio of HLA-DM:HLA-DO as a novel biomarker of response to treatment. Although the sample size was limited, combination therapy with high-dose IPI and high-dose IL-2 was feasible and associated with clinical benefit. IL-2-based compounds in combination with CTLA-4 blockade should be studied in advanced melanoma patients who fail to benefit from first-line PD-1 blockade.
Clinical Trial Registration:ClinicalTrials.gov, NCT02203604. Registered 30 July 2014, https://clinicaltrials.gov/ct2/show/NCT02203604.


Δεν υπάρχουν σχόλια:
Δημοσίευση σχολίου